Due to their widespread presence in the environment and potential health risks, per- and polyfluoroalkyl substances (PFAS) have emerged as a significant topic of concern in recent years. As regulatory agencies work to tighten controls, the introduction of a new federal maximum contaminant level (MCL) for PFAS in drinking water underscores the urgency for effective water treatment solutions. We will explore the implications of the new MCL and discuss the critical role of water treatment providers in addressing PFAS contamination.
Understanding the New MCL for PFAS
The United States Environmental Protection Agency (USEPA) recently published their final Drinking Water Standard for PFAS, which expands on a draft that was first proposed in March 2023 and regulates six types of PFAS. The standard includes conventional MCLs for perfluorooctanoic acid (PFOA), perfulorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA), perfluorohexane sulfonic acid (PFHxS), and hexafluoropropylene oxide dimer acid (HFPO-DA) (commonly known as GenX chemicals). It also includes a hazard index (HI) MCL for mixtures containing two or more of PFNA, PFHxS, HPFO-DA, and perfluorobutane sulfonic acid (PFBA). These MCLs and the hazard index formula are described in the table per the USEPA PFAS National Primary Drinking Regulation.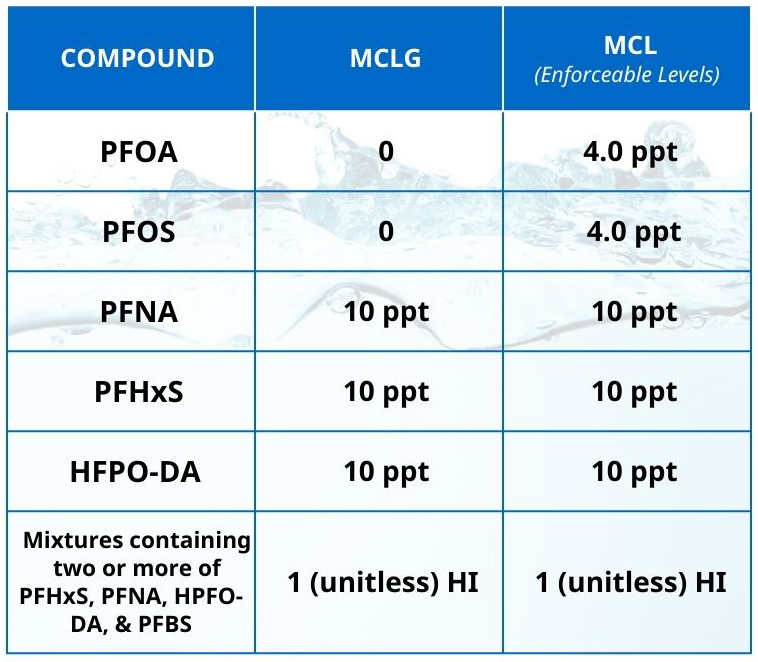
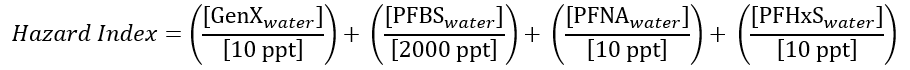
The HI approach is novel for contaminants regulated under the Safe Drinking Water Act, but it has been used elsewhere by the EPA to evaluate groups of contaminants that may have complementary or cumulative health effects. The denominators in the equation above represent the reference dose for each constituent.
The inclusion of analytes beyond PFOA, PFOS, PFNA, PFHxS, and HFPO-DA in the final rule could present greater treatment challenges and drive operational costs. Much of the previous focus on PFAS has centered around PFOA and PFOS, so many communities have already installed treatment equipment following detection through EPA’s Unregulated Contaminant Monitoring Rule (UCMR) 3 or state-driven testing and regulation. However, shorter-chain compounds, like PFBS and HPFO-GA (GenX), are more difficult to remove, and existing systems may struggle to meet the requirements or suffer from the need to change out media more frequently. Moving forward, it is important to assess the efficacy of different treatment options to ensure compliance not just with PFOA and PFOS but the hazard index as well.
Sampling for PFAS
Sampling is important to understand what PFAS analytes are present as well as their concentrations at each water source. Due to the ubiquity of PFAS in consumer products and even materials used in field and laboratory operations, there is a reasonable risk of contamination when collecting samples. Care should be taken to mitigate possible contamination, and it is best practice to include blanks for comparison. For more information, the EPA directs individuals to the Interstate Technology Regulatory Council’s (ITRC) PFAS Fact Sheet that includes a section on sampling and analysis.The EPA has published three methods for analyzing PFAS in drinking water, but for our purposes, we will speak about two specifically:
- Method 537 was finalized in 2009 and covers fourteen PFAS but not all HI analytes
- Method 537.1 expands on 537 to target eighteen PFAS compounds, covering all HI analytes.
- Method 533 was developed to capture shorter chain PFAS and is the most comprehensive, targeting twenty-five analytes.
Background water chemistry is equally important when assessing treatment options, and standard wet chemistry analyses should occur in parallel with PFAS testing to understand potential treatment interferences. Many common PFAS treatment technologies can be sensitive to co-contaminants like iron, manganese, or dissolved organics, and addressing such constituents through a holistic treatment approach can be critical to optimize performance and minimize operating costs. Pre-treatment of such constituents may empower the use of different PFAS treatment technologies that would not be successful alone for varying water sources.
Assessing Treatment Options
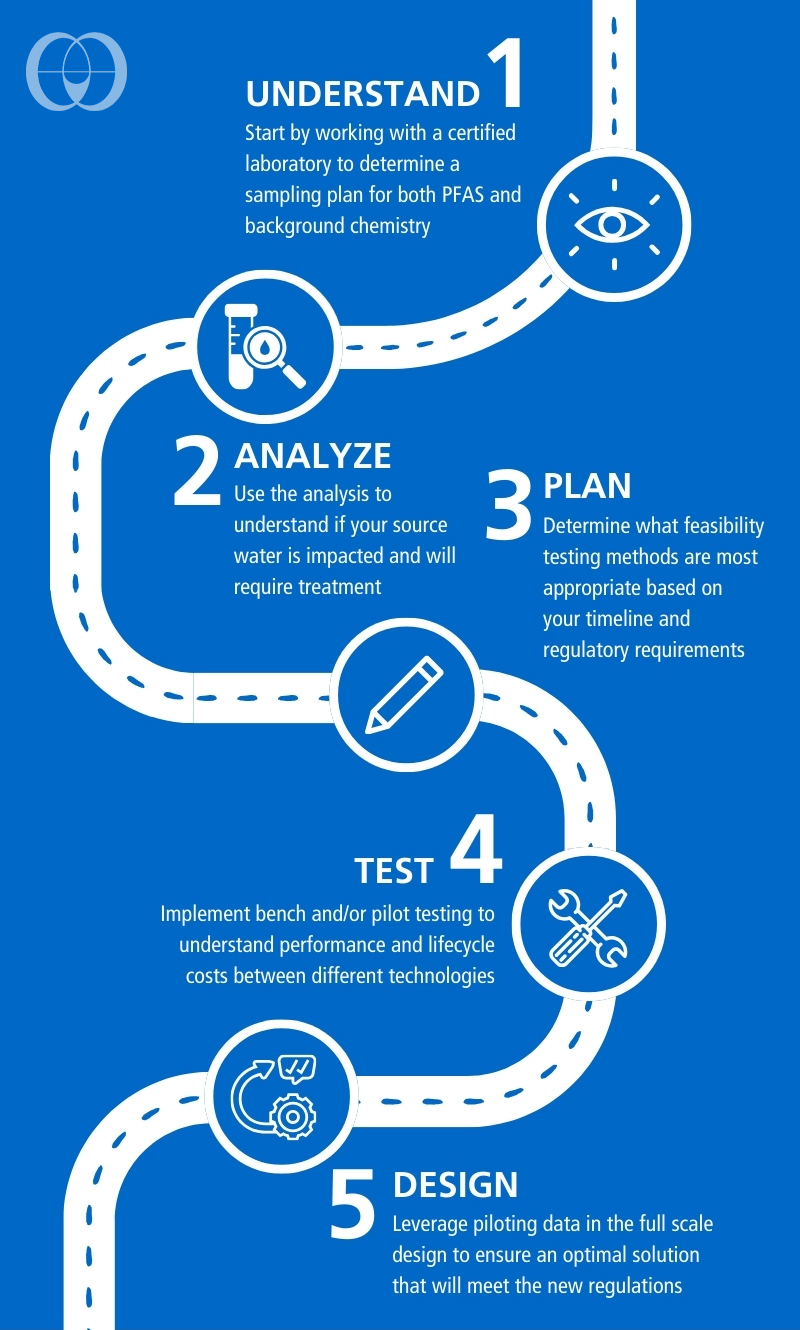 When assessing PFAS treatment options, there are different methods available to ensure reliable treatment and understand the potential operating costs. One method to compare different adsorption technologies is the Rapid Small Scale Column Test (RSSCT). This expedited bench-scale test allows for treating hundreds of thousands of bed volumes in days, whereas a pilot test would take months to exhaust a pilot column on-site. The method was originally developed for granular activated carbon (GAC) but is being applied to ion exchange and other adsorptive media to determine their performance and capacity for PFAS on different source waters. The RSSCT can be a powerful tool for comparing several media options quickly and cost effectively.
When assessing PFAS treatment options, there are different methods available to ensure reliable treatment and understand the potential operating costs. One method to compare different adsorption technologies is the Rapid Small Scale Column Test (RSSCT). This expedited bench-scale test allows for treating hundreds of thousands of bed volumes in days, whereas a pilot test would take months to exhaust a pilot column on-site. The method was originally developed for granular activated carbon (GAC) but is being applied to ion exchange and other adsorptive media to determine their performance and capacity for PFAS on different source waters. The RSSCT can be a powerful tool for comparing several media options quickly and cost effectively.After narrowing the feasible options for treatment, on-site pilot testing is valuable for understanding variations in water chemistry and any effects on long-term performance. Pilot testing may also be required by state regulators for proof of concept. Given the time sensitive nature of complying with the PFAS rule and the durations necessary for pilot testing, design of the full-scale system may need to occur concurrently to keep on schedule.
The newly promulgated treatment standard for PFAS will come with challenges due to the low levels required to stay in compliance. It is important to do due diligence on what is most appropriate for drinking water needs and to leverage resources within regulatory agencies and consulting firms that can assist and advise through this change.
How We Can Help
Tonka Water, a Kurita brand, has been a trusted partner in municipal drinking water treatment for over 65 years, evolving with our customers to meet their growing needs over a changing regulatory landscape. Our team is here to help you navigate the new PFAS rule and work toward compliance. Please reach out to learn more about evaluating different options for your particular situation.Reference List
ITRC (Interstate Technology & Regulatory Council). 2023. PFAS Technical and Regulatory Guidance Document and Fact Sheets. Interstate Technology & Regulatory Council, PFAS Team. https://pfas-1.itrcweb.org/
United States Environmental Protection Agency. (2023, May 30). EPA PFAS Drinking Water Laboratory Methods. https://www.epa.gov/pfas/epa-pfas-drinking-water-laboratory-methods
United States Environmental Protection Agency. (2024, February 1). Fifth Unregulated Contaminant Monitoring Rule. https://www.epa.gov/dwucmr/fifth-unregulated-contaminant-monitoring-rule
United States Environmental Protection Agency. (2024, March 13). Per- and Polyfluoroalkyl Substances (PFAS): PFAS National Primary Drinking Water Regulation. https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas
About the Authors:
 Nicole Bolea, PE has over 10 years of experience in water treatment. She is a professional engineer in the state of Minnesota with a degree in Chemical Engineering and a minor in Environmental Engineering from the University of Minnesota Duluth. She also holds a master’s in business administration from Colorado State University. She began her career in the water industry designing biological wastewater systems focusing on nutrient removal. Moving from design to business development, she has supported all areas of the United States and Canada. She developed, brought to market, and sold technology specifically to treat and remediate PFAS and other emerging contaminants. Now she leads the municipal sales team at Tonka Water, a Kurita brand.
Nicole Bolea, PE has over 10 years of experience in water treatment. She is a professional engineer in the state of Minnesota with a degree in Chemical Engineering and a minor in Environmental Engineering from the University of Minnesota Duluth. She also holds a master’s in business administration from Colorado State University. She began her career in the water industry designing biological wastewater systems focusing on nutrient removal. Moving from design to business development, she has supported all areas of the United States and Canada. She developed, brought to market, and sold technology specifically to treat and remediate PFAS and other emerging contaminants. Now she leads the municipal sales team at Tonka Water, a Kurita brand. T.J. Stroebl is a market development leader at Kurita America, specializing in equipment systems. After earning a chemical engineering degree from the University of Minnesota, he has spent his career with Kurita America focusing on process and equipment design, troubleshooting, and development relevant to water treatment systems. T.J. is an active member of the American Water Works Association (AWWA), currently serving as vice chair on the Manufacturers/Associates Council (MAC).
T.J. Stroebl is a market development leader at Kurita America, specializing in equipment systems. After earning a chemical engineering degree from the University of Minnesota, he has spent his career with Kurita America focusing on process and equipment design, troubleshooting, and development relevant to water treatment systems. T.J. is an active member of the American Water Works Association (AWWA), currently serving as vice chair on the Manufacturers/Associates Council (MAC).
